Concentration measurement in bases
The concentration measurement in bases represents an important aspect in chemical analysis and various production processes. It focuses on determining the concentration of bases in a mixture. This procedure is crucial for accurately assessing the chemical composition and reactivity of bases.
Among the most common bases used in chemistry are: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Ammonia (NH3), Calcium hydroxide (Ca(OH)2), Magnesium hydroxide (Mg(OH)2) and ethanolamines.
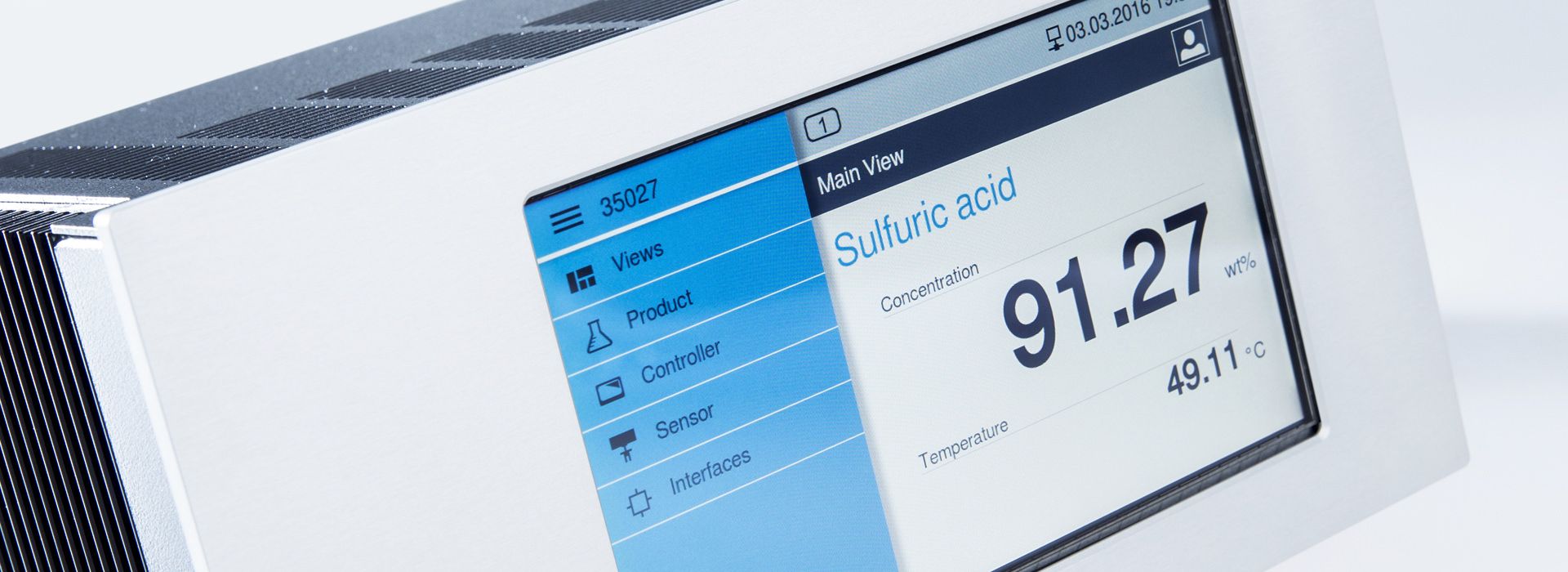
Concentration measurements with LiquiSonic® measuring devices
The LiquiSonic® Measuring systems enable inline concentration measurements of bases. The measurement technology can analyze the components of mixtures and provide information about the concentration or density in real-time. The sensors are based on the principle of ultrasonic speed measurement. Therefore, they are almost maintenance-free and can reliably provide measurement values even under demanding conditions.
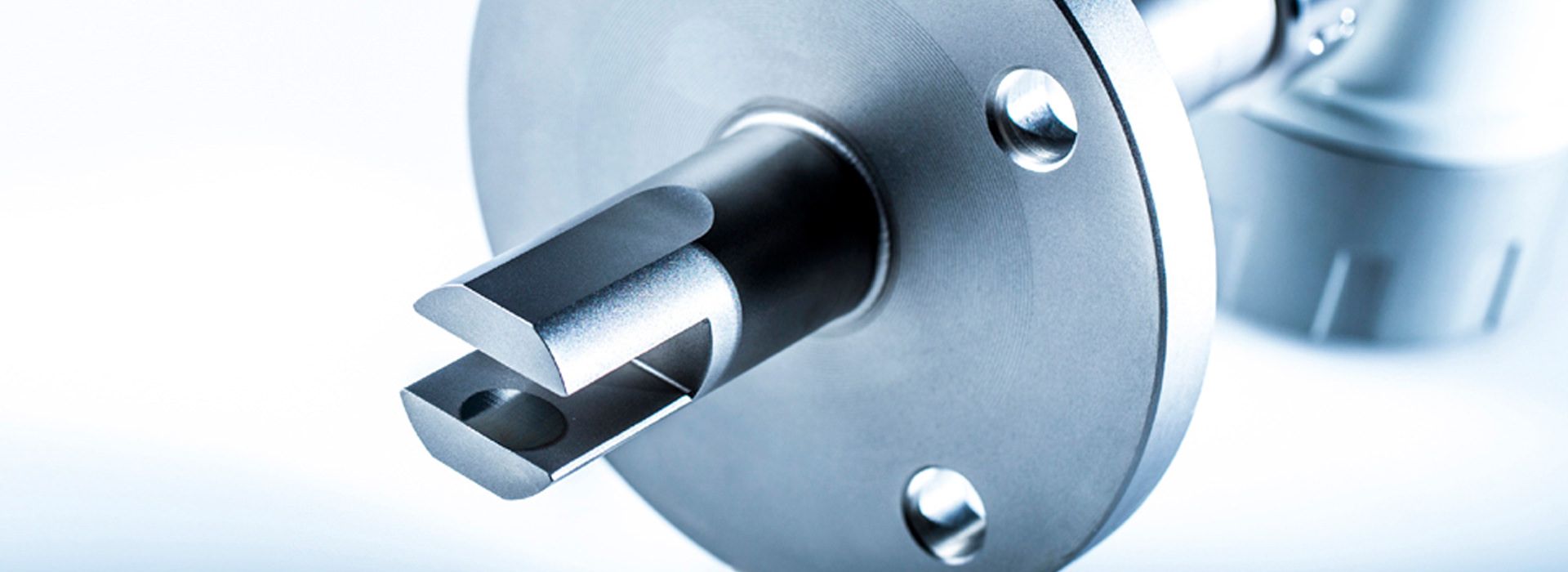
Installation of LiquiSonic® Measuring systems
The LiquiSonic® Sensors can be installed directly without bypass into the user's system, for example in pipelines. There are also various versions tailored to specific applications with special properties.
What is a base?
In chemistry, a base is typically defined as a substance capable of accepting protons (H+), which results in reducing the concentration of free protons in a solution and thereby indirectly reducing the amount of hydronium ions (H3O+). This property of binding protons allows bases to play an essential role in the equilibrium of chemical reactions.
The pH value of a solution serves as a quantitative measure of its acidity; a higher pH value indicates a lower concentration of H+ and thus points to a stronger basicity. It should be noted that according to Lewis, bases can also act as electron pair donors, expanding their definition beyond merely accepting protons.
In industrial processes, the targeted control of the pH value by adding bases is essential to create precise reaction conditions and achieve optimal results. This control allows for a finely tuned adjustment of the reaction environment, which is critical for the efficiency and quality of chemical production.
Which bases can be measured?
In the chemical industry, where the accuracy of base concentrations is crucial, tests are often conducted on substances whose pH values are determined by the concentration of free hydroxide ions - the counterparts of hydronium ions.
Particularly in the field of water treatment applications, such as wastewater treatment or cooling systems, bases like sodium hydroxide or ammonia are used because of their ability to bind hydrogen ions and thereby increase the pH value.
It turns out that the precise determination of these concentrations - through titrimetric or electrochemical methods - is essential to control the corrosive properties of water and optimize the efficiency of processes that are highly pH-dependent.
How is the concentration of a base determined?
The concentration of bases can be determined in various ways. Some common methods are:
- Sound velocity measurement: In this method, the speed at which sound waves travel through a base solution is measured. It is particularly suitable for bases with different molecular sizes and structures, as the speed of sound is influenced by these factors.
- Titration: In titration, the base is mixed with an acid of known concentration until the neutralization point is reached. This method is ideal for precise measurements but unsuitable for bases that do not fully react with the acid or where side reactions occur.
- pH measurement: This method measures the hydroxide ion concentration in the solution to determine the base strength. It is effective in aqueous solutions but unreliable for very strong bases or in the presence of other ions that can affect the pH meter.
- Conductivity measurementHere, the electrical conductivity of the base solution is measured, which depends on the ion concentration. This method is useful for ionic bases but inaccurate for non-ionic or weak bases, as their ions do not sufficiently contribute to conductivity.
- SpectroscopySpectroscopic methods, such as UV-Vis spectroscopy, measure the absorption or emission of light in a base solution. This is suitable for bases that absorb specific wavelengths but not suitable for bases without characteristic absorption bands.
- Density measurementThe density of a base solution can provide information about its concentration. This is particularly effective for pure bases or solutions with a known solvent, but problematic for mixed solutions or when the density is influenced by other dissolved substances.
- Ion chromatographyThis technique separates the ions in a base solution and measures their concentrations. It is particularly useful for complex base mixtures but less effective for simple, single-component base solutions.
Applications of base concentration measurements
In the field of industrial and laboratory-based processes, measuring the concentration of bases becomes an essential activity to draw precise conclusions about the quantity of hydrogen ions (often referred to as hydronium ions) and thus accurately determine the pH value of a solution.
The concentration of a base is particularly relevant in chemical synthesis processes, wastewater treatment, and quality control of pharmaceuticals and food, as its control can influence reaction speed, define the end product, and ensure compliance with safety standards.
Specifically equipped with advanced sensors for detecting hydronium ion concentration, modern analytical devices allow precise pH adjustment, which is indispensable for the successful completion of numerous industrial processes. As a result, the use of these technologies increases efficiency, ensures product quality, and ultimately minimizes environmental impact.