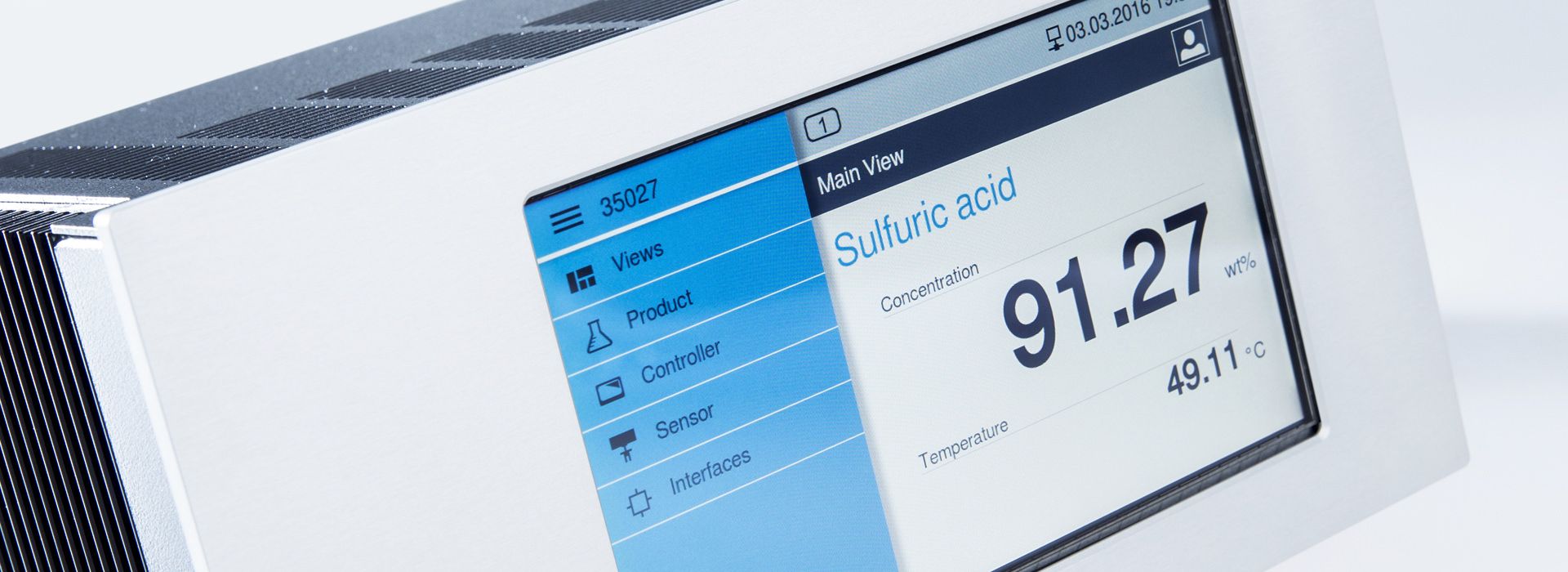
Concentration measurement in liquids
Fundamentals of concentration measurement
The determination of the concentration of various liquids plays a crucial role in numerous procedures of different processes. The ratio of two substances to each other in a mixture or solution is measured and assessed.
A central factor of this concentration measurement is the amount-of-substance concentration. It is defined as the amount of a substance per unit volume and is particularly important in the analysis of solutions. It enables an accurate assessment of the chemical composition and reactivity, making it an indispensable tool in many areas.
Furthermore, there are various measurement ranges that allow the amount-of-substance concentration to be measured in different ways. They significantly expand the possibilities of concentration measurement and increase flexibility with regard to the specific requirements of the mixture or solution to be analyzed.
Finally, the amount of liquid to be analyzed plays an important role. It must be sufficient to allow for accurate measurement, but not so large that it distorts the measurement result or makes the measurement unnecessarily complicated.
An important aspect of concentration measurement is the amount of substance concentration (molarity) in a solution, which is defined as the amount of a substance per unit volume. This is particularly relevant in the analysis of a solution, where the amount of substance concentration is crucial for assessing the chemical composition, concentrations, and reactivity. Accurate measurement of concentrations of an amount of substance in a solution is essential to control processes, ensure quality, and conduct scientificconduct investigations.
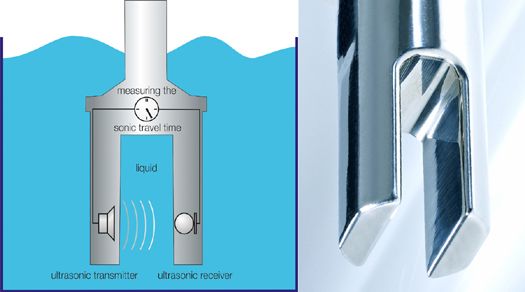
The ultrasonic measurement process of LiquiSonic®
The basis of the measurement process is a time measurement that can be realized very accurately and with long-term stability. From the speed of sound, the concentration or density of a liquid is calculated. Other parameters can also be determined, such as the Brix content, solid content, dry matter, or suspension density.
Our LiquiSonic® Concentration and density measuring devices are used in various processes for the analysis of liquids.
Typically, a calibration curve is determined from the relationship between the speed of sound and the concentration. Based on this, the corresponding concentration is calculated from each measured sound speed value.
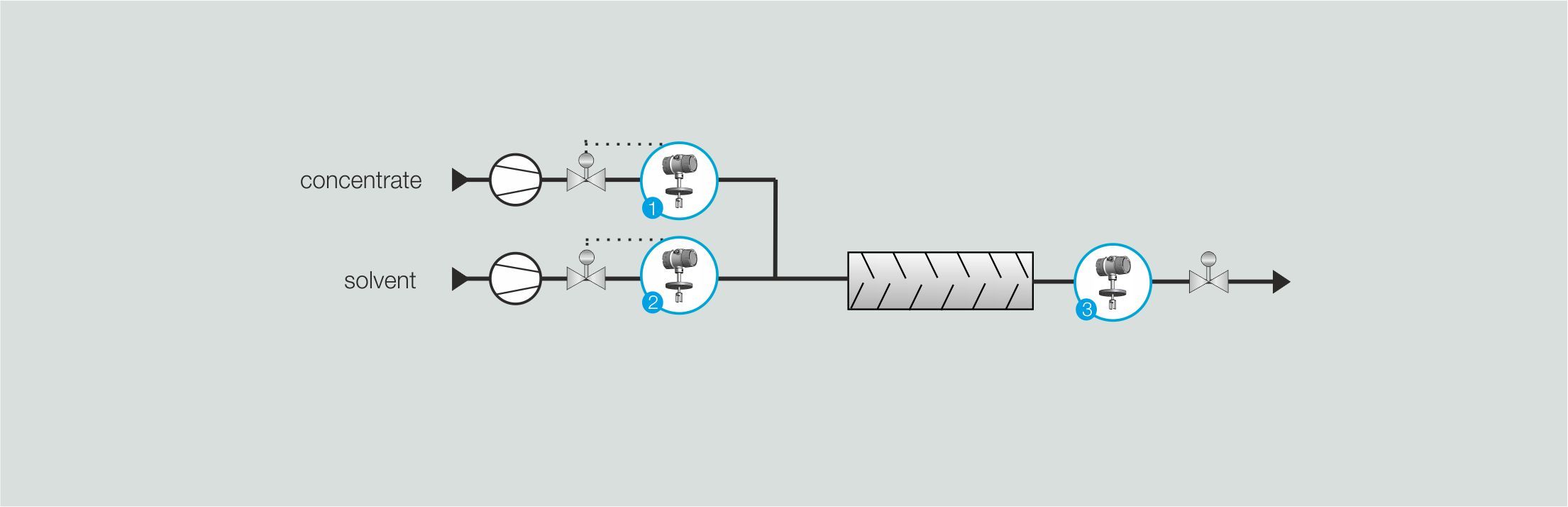
Concentration adjustment
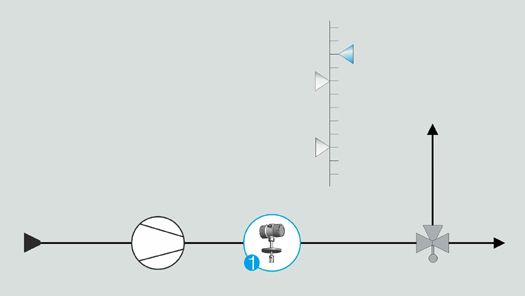
Limit value monitoring
Our ultrasonic measuring devices have no mechanical parts that can wear out or age. They have outstanding advantages over competing measurement methods for determining concentration and density.
High reliability in determining the molar concentration
The measurement process only requires a precise time measurement to determine the molar concentration. The speed of sound is calculated from the transit time and the known distance between the transmitter and receiver. The typical sensor design includes transmitter and receiver in a compact housing.
The measurement process is independent of the conductivity, color, and transparency of the liquid due to the sensors and is characterized by high reliability in determining the molar concentration. The measurement accuracy of the devices is between 0.05 m% and 0.1 m%. In addition to sound speed measurement, all LiquiSonic® sensors have an integrated temperature measurement for temperature compensation in the process.
Applications of concentration measurements
Concentration measurement is one of the essential methods to analyze the quality and safety-relevant features of products and substances. Therefore, it plays a crucial role in several industries. There are various methods for measuring a molar concentration in a solution, depending on the type of substance and the requirements of the application.
A practical example of the application of concentration measurement can be found in the pharmaceutical industry: Here, the precise determination of the concentration of an active ingredient in pharmaceuticals is essential to ensure their effectiveness and safety. This demonstrates the importance of precise measurement methods for determining the concentration of the substance amount in quality assurance.
Further examples:
- Chemistry / Chemical production (For monitoring the composition of mixtures)
- Food production (For controlling the product quality of food)
- Metallurgy (To check the quality of metal ores)
- Environmental analysis (For calculating pollutants in water)
In addition, concentration measurement is also used in many other fields, such as in science.