Other methods for concentration measurement
Alternatives to concentration measurement using sound velocity
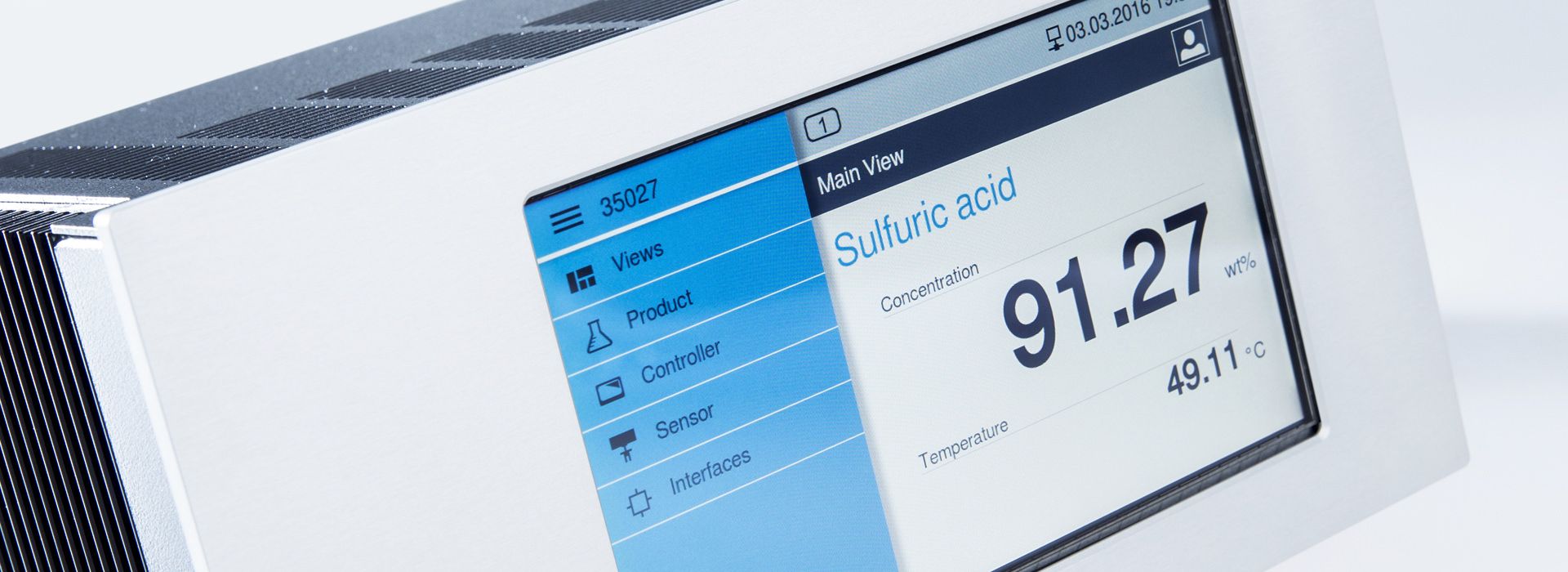
The precise determination of the molar concentration of substances in liquids is crucial for numerous scientific, industrial, and medical applications. Different methods for concentration measurement are used to quantify the exact content of a substance in a specific volume of liquid.
These methods range from spectrophotometric techniques to chromatographic analyses and electrochemical measurements. The choice of the appropriate method depends on the properties of the substance to be analyzed, the requirements of the respective application, and the available resources. There are various methods for measuring the concentration of solutions. Each of these measurement methods for determining molar concentration has its own advantages and disadvantages.
The selection of an appropriate method for concentration measurement in liquids depends on several factors, including:
- Specificity of the application: The type of substances to be measured and the complexity of the solution.
- Accuracy and sensitivity: Required precision and ability to detect a minimum of concentrations.
- Speed and throughput: Need for quick measurement results and ability to handle large sample volumes.
- Cost efficiency: Acquisition and operating costs of the equipment as well as maintenance requirements.
- User-friendliness: Ease of operation and maintenance, especially in environments with little specialized personnel.
Refractometry
The refractometer determines the refractive index of solutions and solid substances to measure concentration. The determination of the refractive index is based on the refraction of light, which is reflected or refracted by a liquid. Depending on the type and concentration of the dissolved substances, the light is refracted differently.
Consequently, the refractive index results from the concentration of the dissolved substances. An optical sensor (window) measures the reflection of a light beam that is reflected by an LED light source after hitting the sample. The refractometry method is extremely sensitive to influencing factors such as vibrations and requires very extensive and time-intensive calibration as well as regular maintenance.
Radiometry
Radiometry uses radioactive radiation to detect concentrations of a substance. A radioactive preparation emits its radiation through the measuring container, which is received by the detector. A scintillator converts the radioactive radiation into flashes of light and evaluates their number. Since the penetration of gamma radiation depends on the substance, the density of the mass is determined from the intensity of the incoming radiation.
Gravimetry
In gravimetry, the measurement of mass concentration is done by measuring the mass of a substance before and after a chemical reaction. It is used to determine the concentration of a specific element or compound in a sample. The basic process for determining the molar concentration includes the steps of precipitation, filtration, and weighing. This method is extremely time-consuming and typically requires large samples. Furthermore, the measurement principle is very prone to errors, as it requires several manual process steps in defining the molar concentration.
Titration
Concentration measurement by titration is done by adding a solution with a known concentration to a solution with an unknown concentration until a chemical reaction occurs. This method is only suitable for certain solutions and due to manual handling prone to errors in the calculation of mass concentration.
Spectrophotometry
In spectrophotometry, the volume of the sample plays a crucial role in determining the volume concentration of a substance. The volume concentration is a measure of the amount of a substance in a mixture relative to the total volume of the mixture. It indicates what proportion of the total volume of a mixture consists of a specific substance.
The light absorption, which is a central measurement in this method, can be significantly influenced by the volume of the sample. Therefore, accurate determination and control of the sample volume is essential for precise measurement results. Spectrophotometry is suitable for a variety of samples, including liquids, gases, and solid materials.
This method for measuring particle volumes is very susceptible to interference factors, which affect the accuracy of the sample.
Chromatography (such as HPLC, GC)
Chromatography separates components of a mixture based on their interactions with a stationary and a mobile phase.