Chlor-Alkali Electrolysis
At the heart of the modern chemical industry is chlor-alkali electrolysis, an indispensable process through which essential raw materials for various applications are obtained. This process technology gains importance not least due to the efficient production of sodium ions (Na+), chloride ions (Cl−), and hydroxide ions (OH−), which are critical raw materials for the production of plastics, pharmaceuticals, and are indispensable in the textile industry.
By applying a direct electric voltage, the chlor-alkali electrolysis separates the sodium chloride solution into elemental chlorine and sodium hydroxide; simultaneously, hydrogen is produced. The functionality of these technically sophisticated electrolysis cells - specifically designed to facilitate the transport of ions while preventing unwanted reactions between the products - is particularly valued in professional circles; because the efficiency and safety of the entire processdepend significantly on precise control mechanisms and the stability of the membrane technologies used.
LiquiSonic® Measuring systems in chlor-alkali electrolysis
The LiquiSonic® measurement technology can be advantageously used in the various process stages of chlor-alkali electrolysis. The customer benefit primarily consists in the Reduction of raw material and energy consumption as well as in the Increase in yield.
LiquiSonic® System
LiquiSonic® is available in three system variants:
LiquiSonic® 20, LiquiSonic® 30 and LiquiSonic® 40.
LiquiSonic® 30 is a high-performance system consisting of a controller with connections for up to four sensors. The sensors can be used at different measuring points.
LiquiSonic® 20 is a variant with reduced functionality and connection for one sensor.
LiquiSonic® 40 enables the simultaneous determination of two concentrations in a mixture. For this purpose, a second physical measurement variable is combined with the sound velocity. In chlor-alkali electrolysis processes, the LiquiSonic® 40 system usually contains a conductivity sensor as the second physical variable.

Measuring principle
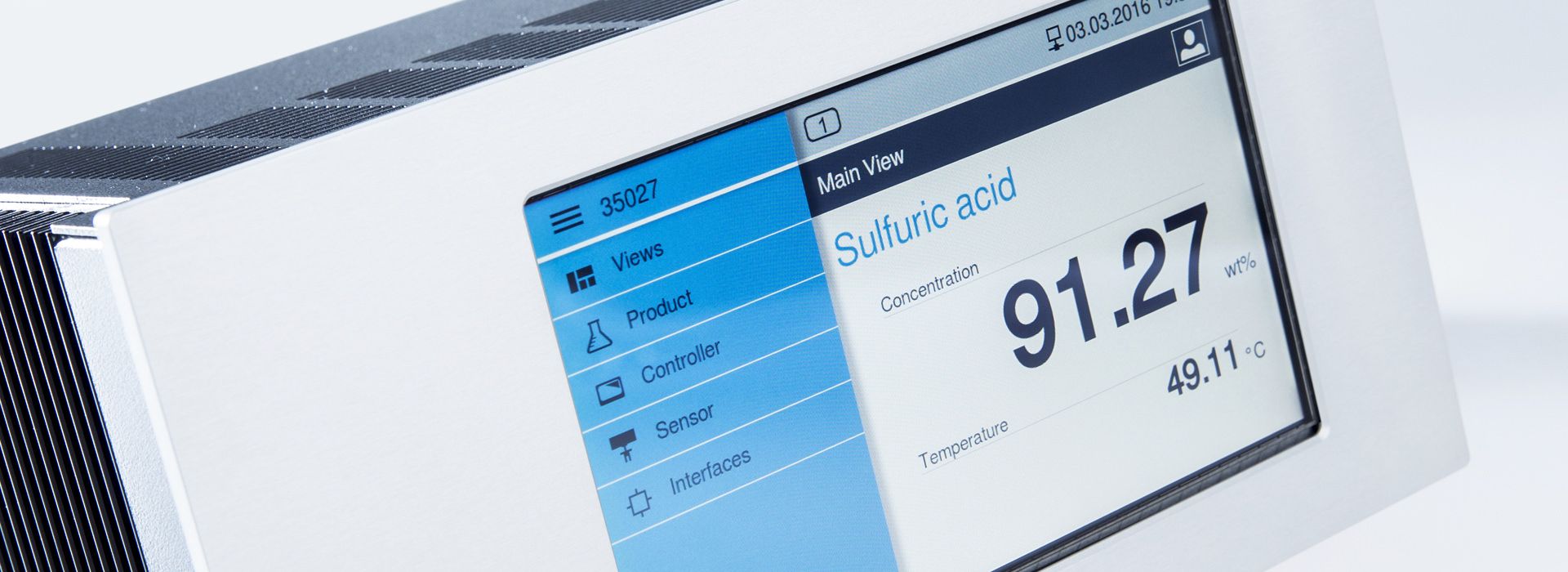
The LiquiSonic® measurement technology analyzes liquid parameters such as concentration or density, detects phase transitions, and is used for reaction monitoring.
The measuring principle is based on the determination of sound velocity in liquids. The distance (d) between the ultrasonic transmitter and receiver is constant due to design, so the sound velocity (v) can be calculated by measuring the transit time (t) (v = d / t). Since the sound velocity depends on the substance concentration, there is a functional relationship through which the concentration can be calculated.
The sound velocity measurement is independent of the liquid's transparency and impresses with high measurement accuracy, reproducibility, and stability. In addition to sound velocity measurement, in the LiquiSonic® Sensor a highly precise and fast temperature measurement for temperature compensation is integrated. For many applications, this offers great advantages over conventional measurement methods.
Sensor
The LiquiSonic® Sensor continuously measures both the concentration and the temperature within the predefined range. The process data is updated every second.
The liquid-contacting sensor component is made of stainless steel or corrosion-resistant material like Hastelloy C-2000, or it is coated with Halar or PFA.
Various additional functions integrated into the sensor, such as the flow monitor (Flow / Stop) or the wet-dry monitoring (full / empty pipeline), complement the process control.
The special LiquiSonic® high-performance technology ensures stable measurement results even with gas bubbles or strong signal attenuation by the process liquid.
Chlor-alkali electrolysis
How does chlor-alkali electrolysis work?
The chlor-alkali electrolysis is an important technical process used for the production of basic chemicals such as chlorine, hydrogen, and caustic soda (sodium hydroxide). An aqueous solution of sodium chloride (salt) is used as an electrolyte. An electrical voltage is applied to the electrodes, which are made of special materials. Through this process, the chloride ions are oxidized to chlorine at the anode, while water is reduced to hydrogen and hydroxide ions at the cathode.Hydroxide ions react with the sodium ions in the solution to form caustic soda. The chlor-alkali electrolysis is a very efficient process used in many industries because it is fast, reliable, and cost-effective, and provides essential chemicals for various industrial applications.
With the help of electric current, the salt (NaCl) into chlorine (Cl2), Caustic soda (NaOH) and hydrogen (H2) is decomposed.
What processes are there in chlor-alkali electrolysis?
Two main processes are used: the diaphragm process and the membrane process.
In both processes, the same electrochemical reaction occurs: the NaCl flows into the anode compartment of the cell, where Cl2 is deposited as chlorine gas. Then the solution proceeds to the cathode compartment, where H2 and NaOH is formed.
Diaphragm process explained:
In the diaphragm process, a porous diaphragm (partition) is used between the anode and cathode. It allows ion exchange but prevents mixing of chlorine and the sodium hydroxide solution. A salt solution is used as the electrolyte, and chlorine is released at the anode, while hydrogen and sodium hydroxide are produced at the cathode. However, the quality of sodium hydroxide in this process is lower than in other methods.
Membrane process explained:
This process uses a special ion-permeable membrane that blocks chlorine ions but allows sodium ions to pass. This leads to the formation of chlorine at the anode and sodium hydroxide and hydrogen at the cathode.
The membrane and diaphragm represent a high cost factor in both processes. The LiquiSonic® measurement technology is used for precise concentration determination of the catholyte to identify and counteract any inefficiencies in the electrolyzer. This ensures an optimal lifespan of the membrane.
Depending on the process used, the catholyte is either an NaOH solution (membrane process) or an NaOH-NaCl solution (diaphragm process). The concentration measurement of the 3-component mixture is realized by using a LiquiSonic® 40 measurement system in which the ultrasound is combined with a conductivity sensor.
Your advantage:
- Maximization of the electrolyzer's efficiency by continuous monitoring of the concentrations in the process
- Energy savings and consumption optimization
- Reduction of complex comparative analyses
- Increase in membrane lifespan
Processing of final products
Caustic soda concentration
Chlor-alkali electrolysis is a process in which sodium chloride (table salt) is converted into chlorine, hydrogen, and caustic soda (sodium hydroxide) under the influence of electrical energy. During this process, sodium ions (Na+) to the cathode, which is negatively charged, and chloride ions (Cl-) to the anode, which is positively charged. At the anode, the oxidation of chloride ions takes place, releasing chlorine. At the cathode, water is reduced to hydrogen and hydroxide ions. These hydroxide ions react with sodium ions to form caustic soda. There are various variants of this process, such as the amalgam process, in which a sodium amalgam is formed at the cathode, which is then further processed into caustic soda, hydrogen, and mercury in a separate stage. Regardless of the process used, theresulting caustic soda is often concentrated by evaporation to achieve a higher concentration.
Marketable Caustic soda (NaOH) usually has a concentration between 45 wt% and 50 wt%. Since the NaOH taken from the electrolysis cells only has a concentration range between 12 wt% and 33 wt%, it is concentrated in multiple-effect evaporators.
Is alongside NaOH also NaCl contained in the solution (diaphragm process), the excess salt crystallizes in the lye during evaporation in the evaporator. This achieves a NaOH concentration between 45 wt% and 50 wt%.
The LiquiSonic® measurement technology continuously determines the concentration of the lye after the evaporator at any time. A subsequent dilution of the caustic soda to a customer-specific product concentration can also be monitored.
Your advantage:
- continuous concentration monitoring of the caustic soda
- reduction of energy costs during evaporation
Chlor gas drying
The drying of chlorine gas is an essential step in the production of chlorine. This process involves the removal of moisture from the chlorine gas to make it suitable for industrial applications. Drying is carried out by physical methods such as cooling and condensing the gas or by using drying agents such as concentrated sulfuric acid or molecular sieves. These techniques ensure that the chlorine is in a pure and dry form. Although chlorine gas drying is a technicallydemanding process, it plays a crucial role in many industries as dried chlorine gas is used for a variety of applications, from water treatment to the production of plastics and pharmaceuticals.
The chlorine gas generated in the anode area of the electrolyzer must be freed from its water content before further use, as its corrosiveness increases with a moisture content above 30 ppm. For drying, the chlorine gas is directed into absorption towers, where the water content in the chlorine gas is absorbed by highly concentrated sulfuric acid (80-99 wt% H2SO4) is absorbed.
The effectiveness of this drying process significantly influences the productivity and quality of the gas. Therefore, reliable measurement of the H2SO4 -concentration is important. The LiquiSonic measurement system® enables continuous and safe monitoring of the H2SO4 -concentration compared to conductivity and density measurement.
Your advantage:
- Elimination of complex sampling
- Continuous monitoring of the H2SO4 -concentration
- Clear signal for concentration determination of H2SO4 between 80 wt% and 100 wt%
- Corrosion prevention through effective drying
Hydrochloric acid production
The chlorine gas generated at the anode of the electrolyzer and the supplied hydrogen form the starting materials for the synthesis of hydrochloric acid. For this purpose, both gases are directed into a burner and react there to form hydrogen chloride. Subsequently, the formed HCl gas flows from the combustion chamber into the integrated isothermal falling film absorber. Here, the gas is absorbed with the help of water or weak acid, forming concentrated hydrochloric acid (37 wt% HCl) is formed.
With the help of LiquiSonic® measurement technology, continuous monitoring of the hydrochloric acid concentration is carried out. This allows deviations from the target concentration to be detected and responded to accordingly.
Your advantage:
- Continuous concentration monitoring of hydrochloric acid (20-40 wt% HCl)
- Ensuring a highly accurate target concentration
Dissolution station and brine purification
The raw product sodium chloride (NaCl) is obtained either by evaporating seawater, mining, or leaching salt deposits (caverns). The raw brine contains impurities and calcium or magnesium salts, which can clog the fine pores of the diaphragm or membrane during electrolysis, significantly reducing their lifespan. For this reason, these impurities are precipitated in agitator tanks (dissolution vessels) by adding caustic soda Sodium hydroxide After precipitation, the impurities are separated using a pressure filter.
The purity of the brine concentration is of particular importance for the subsequent electrolysis. The LiquiSonic® Measuring system ensures a highly precise determination of the brine concentration at any time. Installation takes place in the dissolving station when using mined salts or at the transfer point from the brine supplier in cavern production.
Your advantage:
- Avoidance of quality drops in brine purification
- Increase of membrane lifespan
- Incoming goods inspection (in cavern production)
- Reduction of water or steam consumption (when dissolving the salt)
- Reduction of electrical energy