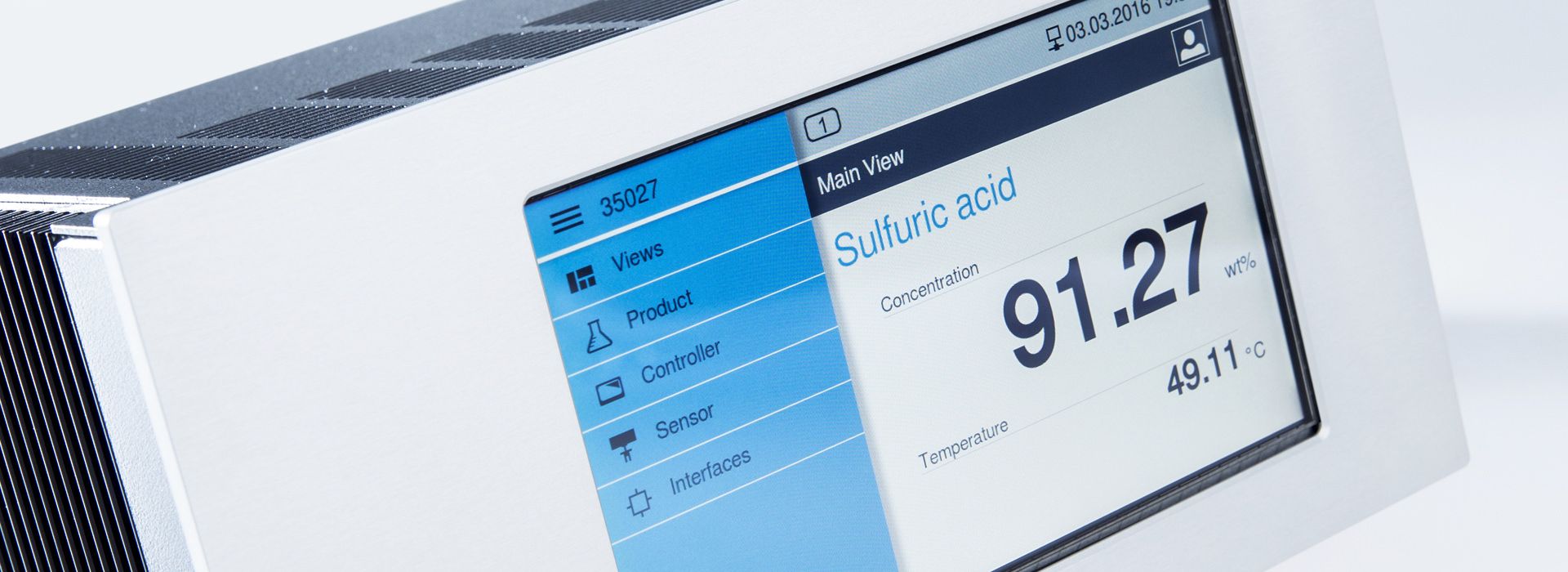
Density measurement in liquids
The density, defined as the measure of mass per volume, plays a central role in the characterization of liquids. A density meter is far more than just a device; it is an indispensable tool for achieving precision in numerous fields. Its applications range from ensuring product quality and control in pharmaceutical production to assisting in the formulation of chemical compounds. In conjunction with an acoustic sensor that responds to changes inliquid composition and concentration, this instrument transforms physical measurements such as mass, volume, and sound velocity into valuable data. This data then serves as a source of information and decision support in various industries.
Innovative approaches in this field are based on principles such as the speed of sound, which provides information about the speed at which sound waves travel through a liquid. This measurement is key to verifying the homogeneity and consistency of a sample. Through detailed analysis of such parameters, professionals can decipher the complex properties of liquids. This also includes understanding their identity and behavior, which is crucial for predicting their behavior under differentconditions and setting standards in the respective industries. By exploring these metrics, the density meter becomes not just a measuring instrument but a beacon for innovation and quality in the development and application of liquids.
The ultrasonic measurement method of LiquiSonic®
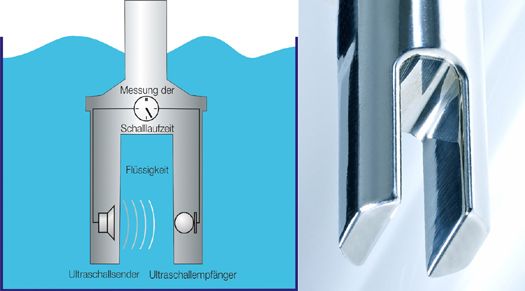
The basis of the measurement method is a time measurement that can be realized very accurately and with long-term stability. From the speed of sound, the concentration or density of a liquid is calculated, which provides information about product quality. Other parameters can also be determined, such as the Brix content, solid content, dry matter, or suspension density.
Our ultrasonic measuring devices have no mechanical parts that can wear out or age. They have outstanding advantages over competing measurement methods for determining concentration and density.
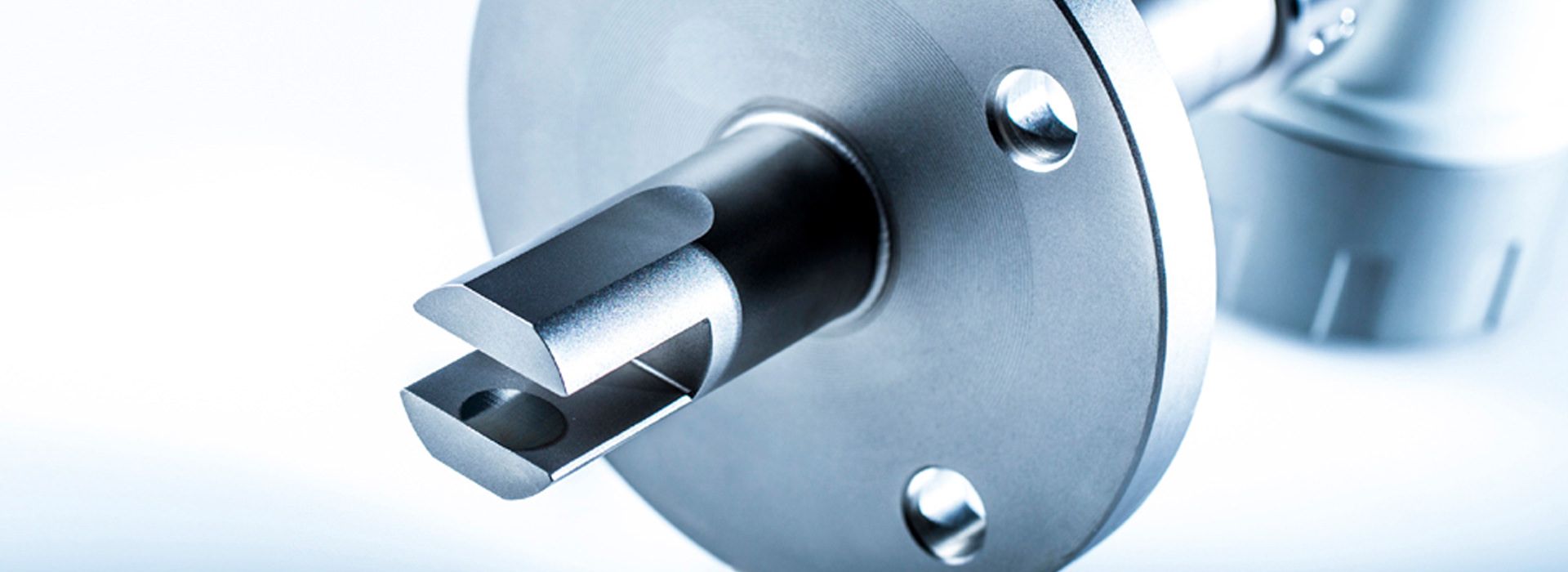
The measurement method only requires precise time measurement. The speed of sound is calculated from the sound transit time and the known distance between the transmitter and receiver. The typical sensor setup includes transmitter and receiver in a compact housing.
The measurement method is independent of the conductivity, color, and transparency of the liquid and is characterized by high reliability. The measurement accuracy of the devices is between 0.05 m% and 0.1 m%. In addition to the speed of sound measurement, all LiquiSonic® sensors have an integrated measurement of the temperature in the process.
Our LiquiSonic® Concentration and density measuring devices are used in various processes for the analysis of liquids.
Typically, a calibration curve is determined from the ratio or relation between the speed of sound and the concentration. Based on this, the corresponding concentration is calculated from each measured speed of sound value.
Basics of density measurement
Density measurements play an important role in one process or another. The mass of a specific substance in a volume is measured. The density is measured in kilograms per cubic meter (kg/m³).
The formula for a simple density measurement of two substances is: ρ (Rho) is equal to the mass m per unit volume V.
As a physical unit, density is influenced by the temperature and pressure of the substances. This is due to the fact that substances expand or contract with a change in temperature. Therefore, a change in temperature has a significant impact on the accuracy of the data in the samples, which is why it is essential for modern sensors to also monitor this component.
The density allows conclusions to be drawn about other chemical and physical properties of a material or substance. Thus, the measurement of density is, for example, an important reference point for quality control.
The density is defined for almost all materials. Due to the wide range of available information, density has become one of the most universal units that can be used in almost any process.
The accuracy of determining density can be significantly affected by various environmental influences. In particular, temperature and pressure play a crucial role as they directly influence the physical states of a material. Temperature fluctuations can lead to expansion or contraction of the substance to be measured, which in turn leads to changes in its density. Similarly, a change in pressure causes a change in density, especially in gases.
Modern density measuring devices take these factors into account by applying temperature and pressure corrections to deliver precise and reliable results.
The accuracy of density determination can be significantly affected by various environmental influences. In particular, temperature and pressure play a crucial role as they directly influence the physical states of a material. Temperature fluctuations can lead to an expansion or contraction of the material to be measured, which in turn results in a change in its density. A change in pressure also causes a change in density, especially in gases.
Modern density measuring devices take these factors into account by applying temperature and pressure corrections to deliver precise and reliable results.
Development of measuring devices for determining density
Modern density measuring devices have made significant technological advances, leading to higher precision, efficiency, and versatility.
Historical measuring devices, such as simple hydrometers or mechanical scales, were heavily dependent on manual work and visual estimates, making them less reliable in precise density measurement.
Today's devices, however, contain advanced technologies such as ultrasonic sensors that measure the speed of sound in a material, or digital pycnometers that calculate volume and mass with the highest accuracy. These devices are capable of performing automated, fast, and highly precise measurements, even under fluctuating environmental conditions.
Furthermore, features such as automatic temperature and pressure compensation help reduce the impact of environmental changes on the measurement, thereby aiding in the determination of specific gravity with increased accuracy. These technical advances in density measuring devices offer a more reliable, efficient, and versatile user experience compared to their historical counterparts.
Comparison to other measurement methods
Compared to alternative measurement methods, such as determining viscosity, the use of a density meter offers universal application advantages and often proves to be simpler and more cost-effective. Viscosity primarily characterizes the flow properties of a liquid, which is crucial in areas where flow behavior and shear forces are important, such as in the food industry or in the production of lubricants. In contrast, specific gravity,measured with a density meter, is the preferred method when it comes to determining the exact composition or quality of a substance.
Density measurement offers a crucial advantage in analyzing substances in situations where conventional methods are insufficient. In confined spaces, for example, the applicability and accuracy of density-based assessments surpass those relying on the refractive index. While these measurements rely on the bending of light as it passes through liquids - requiring calibration and clear paths - density measurement uses a system that can also function in cramped environments.can work effectively. This adaptability makes density measurements an indispensable tool in various fields, including but not limited to chemical analysis and quality control processes. The precision of density measuring devices ensures that professionals can rely on their readings, making them a preferred method for applications requiring both stringent precision and a high level of reliability.
This is particularly important in the chemical and petrochemical industries as well as in pharmaceutical manufacturing. Here, density meters with their sensors for specific gravity provide invaluable information for the identification of substances, quality control, and monitoring of mixing processes. Even at ambient temperatures, a density meter is an indispensable tool in areas that require precise and reliable measurement results.
Applications of density information
Density measurement in liquids is an important procedure in many areas of application. For example, it plays an important role in the chemical and pharmaceutical industries, where the density of liquids is a crucial factor in the production of drugs and chemicals.
In the food and beverage industry, density determination is also used to ensure the quality and consistency of products such as wine, beer, and milk.
In biology and medicine, the density of liquids is used to study cell and tissue cultures as well as sperm motility.
Furthermore, the density of liquids is continuously measured in the petrochemical industry and oil production to enable precise control of production processes. The diverse application areas of density measurement in liquids highlight its relevance and importance in various industrial sectors and for different purposes.
Methods for density measurement
There are various methods used to determine density. Each of these methods has its own advantages and limitations, which is why they are suitable for different applications.
In precision measurement of the density of liquids, especially in industrial applications, the accuracy of the measurement methods used is crucial. This is particularly true for hazardous areas where the presence of flammable materials or vapors requires strict safety protocols. The ability to capture reliable data under such conditions is not only crucial for workplace safety but also significantly contributes to the maintenance ofproduct quality. Accurate density determination allows operating personnel to monitor and control critical process parameters, thereby increasing operational efficiency while minimizing the risk of material losses and potentially dangerous situations.
Hydrometric method for measuring density
This traditional method uses a hydrometer, a special measuring instrument that is immersed in the liquid to be measured. The principle is based on Archimedes' principle: the hydrometer sinks to different depths depending on the density of the liquid. The density can then be read directly on the hydrometer scale. This method is inexpensive and easy to handle, but less accurate and prone to errors due to temperature fluctuations and human reading errors.It is not suitable for viscous liquids or solids and provides more of a qualitative than a quantitative measurement.
Hydrostatic weighing method for determining density
In this method, an object is weighed both in air and in a liquid. The density of the liquid is calculated by relating the buoyancy experienced by the object in the liquid to its weight in the air. This method is accurate and reliable, but requires precise scales and is more time-consuming than other methods. It is particularly suitable for laboratory applications and for materials that require a high degree of accuracy in density measurement.
Radiological measurement of density
In this method, ionizing radiation, usually gamma or X-rays, is used to determine the density of a material. The radiation is passed through the material and a detector measures the attenuation of the radiation. The denser the material, the greater the attenuation. This method is well-suited for inhomogeneous or large objects and allows for non-invasive measurement. However, it requires specialized personnel and strict safety measures due to the use of ionizing radiation.
Pycnometer method for measuring density
A pycnometer is a precisely manufactured vessel with a known volume. To determine the density, the pycnometer is first weighed empty and then filled with the sample. The difference between the weights, divided by the volume of the pycnometer, gives the density of the sample. This method is very accurate and is often used for liquids and fine powders, but is less suitable for large quantities or materials with high viscosity.
Gas pycnometer for determining density
A gas pycnometer uses a gas (usually helium) to determine the density of solids. The sample is placed in a chamber and the volume of gas displaced by the sample is measured. The density is calculated from this volume and the mass of the sample. This method is particularly useful for porous materials or powders and offers high accuracy. However, it is more complex and generally limited to laboratory applications.
Our LiquiSonic® Concentration and density measuring devices are used in various processes for the analysis of liquids.
In a typical case, a calibration curve is determined from the relationship between sound velocity and concentration. Based on this, the corresponding concentration is calculated from each measured sound velocity value.
Density measurements with LiquiSonic®
LiquiSonic® Systems are used in a variety of processes to determine the density of different substances inline and automatically.
Density and sound velocity of some liquids
In the following table, we have listed the density and sound velocity of various liquids that are typically measured and used.
| Liquid | Chemical formula | T [°C] |
| v [m/s] | |
| Acetal | Carbon Hydrogen3Carbon Hydrogen(OC2H5)2 | 24 | 1.03 | 1378 | |
| Acetate ester | Carbon Hydrogen4 CO.Carbon Hydrogen4 Carboxyl2H5 | 25 | 1.021 | 1417 | |
| Acetone | Carbon Hydrogen3CO.Carbon Hydrogen3 | 20 | 0.7992 | 1192 | |
| Acetonedicarboxylic acid | C.(Carbon Hydrogen2Carboxylate2H5)2 | 22 | 1.085 | 1348 | |
| diethyl ester | |||||
| Acetonitrile | Carbon Hydrogen3Cyanide | 20 | 0.783 | 1304 | |
| Acetonylacetone | C6H10O2 | 20 | 0.971 | 1416 | |
| Acetophenone | C6H5.CO.Carbon Hydrogen3 | 20 | 1.026 | 1496 | |
| Acetylacetone | C5H8O2 | 20 | 0.97 | 1383 | |
| Acetyl chloride | C2H3Oxocarbonl | 20 | 1.103 | 1060 | |
| Acetylene dichloride (cis) | Chloromethane = Chloromethane | 25 | 1.262 | 1025 | |
| Acetylene tetrabromide | Bromomethane2. Bromomethane2 | 20 | 2.963 | 1041 | |
| Acetylene tetrachloride | Chloromethane2.Chloromethane2 | 28 | 1.578 | 1155 | |
| Acrolein | C3H4O | 20 | 0.841 | 1207 | |
| Adipic acid diethyl ester | Carbon Hydrogen2.Carbon Hydrogen2.Carboxylate2H5 | 22 | 1.013 | 1376 | |
| | | |||||
| Carbon Hydrogen°2Carbon Hydrogen2.Carboxylate2H5 | |||||
| Adipic acid dimethyl ester | Carbon Hydrogen2Carbon Hydrogen2CarboxylateH3 | 22 | 1.067 | 1469 | |
| | | |||||
| Carbon Hydrogen2Carbon Hydrogen2CarboxylateH3 | |||||
| Ammonium nitrate 10% | NH4Nitric Oxide3 | 20 | 1540 | ||
| Allyl chloride | Carbon Hydrogen2Carbon Hydrogen . Carbon Hydrogen2CChlorine | 28 | 0.937 | 1088 | |
| Formic acid | Formic acid | 20 | 1.212 | 1287 | |
| Amyl ether (iso) | C5H11Oxocarbon5H11 | 26 | 0.774 | 1153 | |
| Amyl alcohol (n) | C5H11Hydroxyl | 20 | 0.816 | 1294 | |
| Amyl alcohol (tert.) | (Carbon Hydrogen3)2C(Hydroxyl)C2H5 | 28 | 0.809 | 1204 | |
| Amyl acetate | Carbon Hydrogen3Carboxylate5H11 | 26 | 0.875 | 1168 | |
| Amyl bromide (n) | C5H11Bromine | 20 | 1.223 | 981 | |
| Amyl formate | HCarboxylate5H11 | 26 | 0.869 | 1201 | |
| Aniline | C6H5NH2 | 20 | 1.022 | 1656 | |
| Ascorbic acid 30% | C6H8O6 | 20 | 1578 | ||
| Barium sulfide 120 g/l | BaS | 50 | 1591 | ||
| Benzaldehyde | C7H6O | 20 | 1.046 | 1479 | |
| Benzene | C6H6 | 20 | 0.878 | 1326 | |
| Benzoyl chloride | C6H5Carboxylatel | 28 | 1.211 | 1318 | |
| Benzyl acetone | C10H12O | 20 | 0.989 | 1514 | |
| Benzyl alcohol | C7H7Hydroxyl | 20 | 1.045 | 1540 | |
| Benzyl chloride | C7H7Chlorine | 20 | 1.098 | 1420 | |
| Diethyl succinate | (Carbon Hydrogen2-Carboxylate2H5)2 | 22 | 1.039 | 1378 | |
| Boric acid 5% | H3BO3 | 30 | 1520 | ||
| Pyruvic acid | CMethoxy3Carboxyl | 20 | 1.267 | 1471 | |
| Bromineomal | C2HOBromine3 | 20 | 2.55 | 966 | |
| Bromonaphthalene (a) | C10H7Bromine | 20 | 1.487 | 1372 | |
| Bromineomoform | Bromomethane3 | 20 | 2.89 | 928 | |
| Butyric acid | C3H7Carboxyl | 20 | 0.959 | 1203 | |
| Butyl alcohol (n) | C4H9Hydroxyl | 20 | 0.81 | 1268 | |
| Butyl alcohol (iso) | (Carbon Hydrogen3)2Carbon HydrogenCarbon Hydrogen2Hydroxyl | 20 | 0.802 | 1222 | |
| Butyl alcohol (tert) | C4H10O | 20 | 0.789 | 1155 | |
| Butyl acetate (n) | Carbon Hydrogen3Carboxylate4H9 | 26 | 0.871 | 1271 | |
| Butyl bromide (n) | Carbon Hydrogen3(Carbon Hydrogen2)2Carbon Hydrogen2Bromine | 20 | 1.275 | 990 | |
| Butyl chloride (n) | C4H9Chlorine | 20 | 0.884 | 1133 | |
| 2,3 Butylene glycol | C4H10O2 | 25 | 1.019 | 1484 | |
| Butyl formate | HCarboxylate4H9 | 24 | 0.906 | 1199 | |
| Butyl iodide (n) | Carbon Hydrogen3(Carbon Hydrogen2)2Carbon Hydrogen2J | 20 | 1.614 | 977 | |
| Butyllithium | 20 | 1390 | |||
| Caprolactam | C6H11Nitric Oxide | 120 | 1330 | ||
| Caproic Acid | C5H11Carboxyl | 20 | 0.929 | 1280 | |
| Caprylic Acid | C7H15Carboxyl | 20 | 0.91 | 1331 | |
| Carvacrol | C10H14O | 20 | 0.976 | 1475 | |
| Quinaldine | C10H9N | 20 | 1.069 | 1575 | |
| Quinoline | C9H7N | 20 | 1.093 | 1600 | |
| Chlorobenzene | C6H5Chlorine | 20 | 1.107 | 1291 | |
| Ethyl Chloroacetate | Carbon Hydrogen2Chloroformate2H5 | 26 | 1.16 | 1234 | |
| Methyl Chloroacetate | Carbon Hydrogen2Chloroacetate3 | 26 | 1.232 | 1331 | |
| Alpha-Chloronaphthalene | C10H7Chlorine | 20 | 1481 | ||
| Chloroform | Chloromethane3 | 20 | 1.489 | 1005 | |
| Ortho-Chlorotoluene | C7H7Chlorine | 20 | 1.085 | 1344 | |
| Meta-Chlorotoluene | C7H7Chlorine | 20 | 1.07 | 1326 | |
| Para-Chlorotoluene | C7H7Chlorine | 20 | 1.066 | 1316 | |
| Cinnamaldehyde | C9H8O | 25 | 1.112 | 1554 | |
| Citral | C10H16O | 20 | 0.859 | 1442 | |
| Crotonaldehyde | C4H6O | 20 | 0.856 | 1344 | |
| Cyclohexane | C6H12 | 20 | 0.779 | 1284 | |
| Cyclohexaneol | C6H12O | 20 | 0.962 | 1493 | |
| Cyclohexanone | C6H10O | 20 | 0.949 | 1449 | |
| Cyclohexene | C6H10 | 20 | 0.811 | 1305 | |
| Cyclohexylamine | C6H13N | 20 | 0.896 | 1435 | |
| Cyclohexyl Chloride | C6H11Chlorine | 20 | 0.937 | 1319 | |
| Cyclopentadiene | C5H6 | 20 | 0.805 | 1421 | |
| Cyclopentanone | C5H#O | 24 | 0.948 | 1474 | |
| 1-Decene | C10H20 | 20 | 0.743 | 1250 | |
| Decyl Alcohol (n) | C10H21Hydroxyl | 20 | 0.829 | 1402 | |
| Decyl Chloride (n) | C10H21Chlorine | 20 | 0.866 | 1318 | |
| Diacetone Sorbose 50% | 50 | 1557 | |||
| Diacetyl | C4H6O2 | 25 | 0.99 | 1236 | |
| Diethylaniline | C6H5Amino Group2H5)2 | 20 | 0.934 | 1482 | |
| Diethylene Glycol | C4H10O3 | 25 | 1.116 | 1586 | |
| Diethylene Glycol Ethyl Ether | C6H14O3 | 25 | 0.988 | 1458 | |
| Diethyl Ketone | C2H5Carboxylate2H5 | 24 | 0.813 | 1314 | |
| Cis-Dibromomethylene | Bromomethane . Bromomethane | 20 | 2.246 | 957 | |
| Trans-Dibromomethylene | Bromomethane . Bromomethane | 20 | 2.231 | 936 | |
| Dichloroethane | C2H4Chlorine2 | 20 | 1.253 | 1034 | |
| Cis-Dichloroethylene | Dichloromethane | 20 | 1.282 | 1090 | |
| Trans-Dichloroethylene | Dichloromethane | 20 | 1.257 | 1031 | |
| Meta-Dichlorobenzene | C6H4Chlorine2 | 28 | 1.285 | 1232 | |
| Dichlorobenzene (o) | C6H4Chlorine2 | 20 | 1.305 | 1295 | |
| Diethylene glycol diethyl ether | O(Carbon Hydrogen2Carboxylate2H5)2 | 22 | 1.433 | 1435 | |
| Dimethylamine, DMA 60% | (Carbon Hydrogen3)2NH | 20 | 0.826 | 1430 | |
| Dimethylaniline | C8H11N | 20 | 0.956 | 1509 | |
| Dimethylacetamide 90% | C4H9Nitric Oxide | 20 | 0.94 | 1550 | |
| Dimethyl benzoate | |||||
| Dimethylformamide, DMF | C3H7Nitric Oxide | 20 | 0.948 | ||
| Dimethylglutaric acid | C(Carbon Hydrogen3)2Carboxylate2H)2 | 24 | 1.038 | 1371 | |
| dimethyl ester | |||||
| Dioxane | C4H8O2 | 20 | 1.038 | 1389 | |
| Dipentene | C10H16 | 24 | 0.864 | 1328 | |
| Diphenyl ether | C6H5Oxocarbon6H5 | 24 | 1.072 | 1469 | |
| Diphenylmethane | C6H5 - CH2 - C6H5 | 28 | 1.006 | 1501 | |
| Di-n-propyl ether | C6H14O | 20 | 0.747 | 1112 | |
| n-Dodecyl alcohol | C12H25Hydroxyl | 30 | 0.827 | 1388 | |
| Iron(II) sulfate | FeSO4 | 20 | 1.9 | ||
| Elaidic acid | C18H34O2 | 45 | 0.873 | 1346 | |
| Acetic acid | Carbon Hydrogen3Carboxyl | 20 | 1.049 | 1150 | |
| Acetic anhydride | (Carbon Hydrogen3CO)2O | 24 | 1.975 | 1384 | |
| Ethyl ether | C4H10O | 20 | 0.714 | 1008 | |
| Ethanol | C2H5Hydroxyl | 20 | 0.789 | 1180 | |
| Ethyl acetate | Carbon Hydrogen3Carboxylate2H5 | 20 | 0.9 | 1176 | |
| Ethylene oxide | C2H4O | 26 | 0.892 | 1575 | |
| Ethylbenzene | C6H5.C2H5 | 20 | 0.868 | 1338 | |
| Ethylbenzylaniline | C15H17N | 20 | 1.029 | 1586 | |
| Ethyl bromide | C2H5Bromine | 28 | 1.428 | 892 | |
| Ethyl butyrate | C3H7 . Carboxylate2H5 | 24 | 0.877 | 1171 | |
| Ethyl caprylate | Carbon Hydrogen3(Carbon Hydrogen2)6Carboxylate2H5 | 28 | 0.872 | 1263 | |
| Ethylene bromide | C2H4Bromine2 | 20 | 2.056 | 1009 | |
| Ethylene chloride | Carbon Hydrogen2Chlorine . Carbon Hydrogen2Chlorine | 23 | 1.255 | 1240 | |
| Ethylene glycol | C2H6O2 | 20 | 1.115 | 1616 | |
| Ethyleneimine | C2H5N | 24 | 0.8321 | 1395 | |
| Ethyl formate | H . Carboxylate2H5 | 24 | 1.103 | 1721 | |
| Ethyl iodide | C2H5J | 20 | 1.94 | 869 | |
| Ethyl carbonate | CO(Oxocarbon2H5)2 | 28 | 0.977 | 1173 | |
| Ethyl phenyl ketone | C9H10O | 20 | 1.009 | 1498 | |
| Ethyl phthalate | C6H4Carboxylate2H5)2 | 23 | 1.121 | 1471 | |
| Ethyl propionate | C2H5Carboxylate2H5 | 23 | 0.884 | 1185 | |
| Hydrofluoric acid | HF | 0 | 1.2 | 1362 | |
| Formaldehyde 60% | Carbon Hydrogen2O | 85 | 1.103 | 1516 | |
| Formamide | Carbon Hydrogen3Nitric Oxide | 20 | 1.139 | 1550 | |
| Fumaric acid | C4H4O4 | 20 | 1.051 | 1303 | |
| Furfuryl alcohol | C5H6O2 | 25 | 1.135 | 1450 | |
| Geranyl acetate | C12H20O2 | 28 | 0.915 | 1328 | |
| Glycerin | C3H8O3 | 20 | 1.261 | 1923 | |
| Hemelithol | C9H12 | 20 | 0.887 | 1372 | |
| Heptane (n) | C7H16 | 20 | 0.684 | 1162 | |
| Heptanone | C7H14O | 20 | 0.814 | 1207 | |
| 1-Heptene | C7H14 | 20 | 0.699 | 1128 | |
| Heptyl alcohol (n) | C7H15Hydroxyl | 20 | 0.823 | 1341 | |
| Hexamethylene | 20 | 1.201 | 2060 | ||
| diaminodipinate | |||||
| Hexane | C6H14 | 20 | 0.654 | 1083 | |
| Hexyl alcohol (n) | C6H13Hydroxyl | 20 | 0.82 | 1322 | |
| Hexyl chloride (n) | C6H13Chlorine | 20 | 0.872 | 1221 | |
| Hexyl iodide (n) | C6H13J | 20 | 1.441 | 1081 | |
| Hydrindene | C9H10 | 20 | 0.91 | 1403 | |
| Indene | C9H8 | 20 | 0.998 | 1475 | |
| Isopropylbenzene (Cumene) | C6H5Carbon Hydrogen(Carbon Hydrogen3)2 | 20 | 0.878 | 1342 | |
| Iodobenzene | C6H5J | 20 | 1.83 | 1113 | |
| Ionone A | C13H20O | 20 | 0.932 | 1432 | |
| Carbolic acid | C6H5Hydroxyl | 20 | 1.071 | 1520 | |
| Kerosene | 20 | 0.81 | 1301 | ||
| Cresol (o) | C7H8O | 25 | 1.046 | 1506 | |
| Cresyl ethyl ether (o) | C6H4(Carbon Hydrogen3)Oxocarbon2H5 | 25 | 0.944 | 1315 | |
| Cresyl methyl ether (m) | C6H4Carbon Hydrogen3 Methoxy3 | 26 | 0.976 | 1385 | |
| Linseed oil | 31 | 0.922 | 1772 | ||
| Linalool | C10H17Hydroxyl | 20 | 0.863 | 1341 | |
| Lithium bromide | LiBromine | 20 | 1612 | ||
| Lithium chloride | LiChlorine | 20 | 2.068 | ||
| Maleic acid | C4H4O | 20 | 1.068 | 1352 | |
| Diethyl malonate | Carbon Hydrogen2Carboxylate2H5)2 | 22 | 1.05 | 1386 | |
| Mesitylene | C6H3(Carbon Hydrogen3)2 | 20 | 0.863 | 1362 | |
| Mesityl oxide | C6H10°O | 20 | 0.85 | 1310 | |
| Methyl ethyl ketone | C4H8O | 20 | 0.805 | 1207 | |
| Methyl alcohol | Carbon Hydrogen3Hydroxyl | 20 | 0.792 | 1123 | |
| Methyl acetate | Carbon Hydrogen3CarboxylateH3 | 25 | 0.928 | 1154 | |
| N-Methylaniline | C7H9N | 20 | 0.984 | 1586 | |
| Methyldiethanolamine, MDEA | C5H13Nitric Oxide2 | 20 | 1.04 | 1572 | |
| Methylene bromide | Carbon Hydrogen2Bromine2 | 24 | 2.453 | 971 | |
| 2-Methylbutanol | C5H11Hydroxyl | 30 | 0.806 | 1225 | |
| Methylene chloride | Carbon Hydrogen2Chlorine2° | 20 | 1.336 | 1092 | |
| Methylene iodide | Carbon Hydrogen2J2 | 24 | 3.233 | 977 | |
| Methylene hexaline | C6H10(Carbon Hydrogen3)Hydroxyl | 22 | 0.913 | 1528 | |
| Methyl hexyl ketone | Carbon Hydrogen3COxocarbon6H13 | 24 | 0.817 | 1324 | |
| Methyl isopropyl benzene (p) | C6H4Carbon Hydrogen3Carbon Hydrogen(Carbon Hydrogen3)2 | 28 | 0.857 | 1308 | |
| Methyl isobutyl ketone, MIBK | C6H12O | 20 | 0.8 | 1220 | |
| Methyl iodide | Carbon Hydrogen3J | 20 | 2.279 | 834 | |
| Methyl propionate | C2H5CarboxylateH3 | 24 | 0.911 | 1215 | |
| Methyl silicone | 20 | 1030 | |||
| Methyl cyclohexane | C7°H14 | 20 | 0.764 | 1247 | |
| Methyl cyclohexanol (o) | C7H14O | 26 | 0.922 | 1421 | |
| Methyl cyclohexanol (m) | C7H14O | 26 | 0.914 | 1406 | |
| Methyl cyclohexanol (p) | C7H14O | 26 | 0.92 | 1387 | |
| Methyl cyclohexanone (o) | C7H12O | 26 | 0.924 | 1353 | |
| Methyl cyclohexanone (p) | C7H12O | 26 | 0.913 | 1348 | |
| Monochloronaphthalene | C10H7Chlorine | 27 | 1.189 | 1462 | |
| Monomethylamine, MMA 40% | Carbon Hydrogen5N | 20 | 0.9 | 1765 | |
| Morpholine | C4H9Nitric Oxide | 25 | 1 | 1442 | |
| Sodium hydroxide | NaHydroxyl | 20 | 1.43 | 2440 | |
| Sodium hypochlorite | NaOxocarbonl | 20 | 1.22 | 1768 | |
| Sodium iodide | NaJ | 50 | 1510 | ||
| Nicotine | C10H14N2 | 20 | 1.009 | 1491 | |
| Nitroethyl alcohol | Nitric Oxide2C2H4Hydroxyl | 20 | 1.296 | 1578 | |
| Nitrobenzene | C6H5Nitric Oxide2 | 20 | 1.207 | 1473 | |
| Nitromethane | Carbon Hydrogen3Nitric Oxide2 | 20 | 1.139 | 1346 | |
| Nitrotoluene (o) | Carbon Hydrogen3C6H4Nitric Oxide2 | 20 | 1.163 | 1432 | |
| Nitrotoluene (m) | Carbon Hydrogen3C6H4Nitric Oxide2 | 20 | 1.157 | 1489 | |
| Nonane | C9H20 | 20 | 0.738 | 1248 | |
| 1-Nonene | C9H18 | 20 | 0.733 | 1218 | |
| Nonyl alcohol (n) | C9H19Hydroxyl | 20 | 0.828 | 1391 | |
| Oleic acid (cis) | C18H34O2 | 45 | 0.873 | 1333 | |
| Pelargonic acid | C6H13Carboxyl | 20 | 0.922 | 1312 | |
| Octane (n) | C8H18 | 20 | 0.703 | 1197 | |
| 1-Octene | C8H16 | 20 | 0.718 | 1184 | |
| Octyl alcohol (n) | C8H17Hydroxyl | 20 | 0.827 | 1358 | |
| Octyl bromide (n) | C8H17Bromine | 20 | 1.166 | 1182 | |
| Octyl chloride (n) | C8H17Chlorine | 20 | 0.872 | 1280 | |
| Olive oil | 32 | 0.904 | 1381 | ||
| Diethyl oxalate | Carboxylate2H5)2 | 22 | 1.075 | 1392 | |
| Paraldehyde | C6H12O3 | 20 | 0.994 | 1204 | |
| Pentane | C5H12 | 20 | 0.621 | 1008 | |
| Pentachloroethane | C2Hydrochloric Acid5 | 20 | 1.672 | 1113 | |
| 1-Pentadecene | C15H30 | 20 | 0.78 | 1351 | |
| Perchloroethylene | C2Chlorine4 | 20 | 1.614 | 1066 | |
| Phenethyl ether (Phenetole) | C6H5Oxocarbon2H5 | 26 | 0.774 | 1153 | |
| Pentane | C5H12 | 20 | 0.621 | 1008 | |
| Petroleum | 34 | 0.825 | 1295 | ||
| Benzyl alcohol | C8H9Hydroxyl | 30 | 1.012 | 1512 | |
| Phenylhydrazine | C6H8N2 | 20 | 1.098 | 1738 | |
| Anisole | C6H5Methoxy3 | 26 | 1.138 | 1353 | |
| 3-Phenylpropyl alcohol | C9H11Hydroxyl | 30 | 0.994 | 1523 | |
| Phenyl mustard oil | C6H5Thiocyanate | 27 | 1.131 | 1412 | |
| Picoline (a) | C5H4Nitrogen Carbon Hydrogen3 | 28 | 0.951 | 1453 | |
| Picoline (b) | Carbon Hydrogen3C5H4N | 28 | 0.952 | 1419 | |
| Pinene | C10H16 | 24 | 0.778 | 1247 | |
| Piperidine | C5H11N | 20 | 0.86 | 1400 | |
| Phosphoric acid 50% | H3Phosphorus Oxygen4 | 25 | 1.3334 | 1615 | |
| Polyvinyl acetate, PVAc | 24 | 1458 | |||
| n-Propionitrile | C2H5Cyanide | 20 | 0.787 | 1271 | |
| Propionic acid | Carbon Hydrogen3Carbon Hydrogen2Carboxyl | 20 | 0.992 | 1176 | |
| n-Propyl alcohol | C3H7Hydroxyl | 20 | 0.804 | 1223 | |
| Isopropyl alcohol | C3H7Hydroxyl | 20 | 0.786 | 1170 | |
| Propyl acetate | Carbon Hydrogen3Carboxylate3H7 | 26 | 0.891 | 1182 | |
| n-Propyl chloride | C3H7Chlorine | 20 | 0.89 | 1091 | |
| Propylene glycol | C3H8O2 | 20 | 1.432 | 1530 | |
| Propyl iodide | C3H7J | 20 | 1.747 | 929 | |
| Pseudobutyl-m-xylene | C12H18 | 20 | 0.868 | 1354 | |
| Pseudocumene | C9H12 | 20 | 0.876 | 1368 | |
| Phthalic anhydride | C6H4-(CO)2O | 20 | 1.527 | ||
| Pyridine | C6H5N | 20 | 0.982 | 1445 | |
| Mercury | Hg | 20 | 13.595 | 1451 | |
| Resorcinol dimethyl ether | C6H4(Methoxy3)2 | 26 | 1.054 | 1460 | |
| Resorcinol monomethyl ether | C6H4OH Methoxy3 | 26 | 1.145 | 1629 | |
| Salicylaldehyde | Hydroxyl C6H4Carbon HydrogenO | 27 | 1.166 | 1474 | |
| Methyl salicylate | HydroxylC6H4CarboxylateH3 | 28 | 1.18 | 1408 | |
| Hydrochloric acid 35% | Hydrochloric Acid | 20 | 1.1738 | 1510 | |
| Carbon disulfide | CS2 | 20 | 1.263 | 1158 | |
| Sulfuric acid 90% | H2SO4 | 20 | 1.814 | 1455 | |
| Tetraethylene glycol | C8H18O5 | 25 | 1.123 | 1586 | |
| Tetrabromomethane | C2H2Bromine4 | 20 | 2.963 | 1041 | |
| Tetrachloroethane | C2H4Chlorine | 20 | 1.6 | 1171 | |
| Tetrachloroethylene | C2Chlorine4 | 28 | 1.623 | 1027 | |
| Carbon tetrachloride | CChlorine4 | 20 | 1.595 | 938 | |
| Tetrahydrofuran, THF | C4H8O | 20 | 0.889 | 1304 | |
| Tetralin | C10H12 | 20 | 0.967 | 1492 | |
| Tetranitromethane | Cyanide4O8 | 20 | 1.636 | 1039 | |
| Thiodiglycolic acid diethyl ester | S(Carbon Hydrogen2Carboxylate2H5)2 | 22 | 1.142 | 1449 | |
| Thioacetic acid | C2H4OS | 20 | 1.064 | 1168 | |
| Thiophene | C4H4S | 20 | 1.065 | 1300 | |
| Toluidine (o) | C7H9N | 20 | 0.998 | 1634 | |
| Toluidine (m) | C7H9N | 20 | 0.989 | 1620 | |
| Toluene | C7H8 | 20 | 0.866 | 1328 | |
| Transformer oil | 32 | 0.895 | 1425 | ||
| Triethylene glycol | C6H14O4 | 25 | 1.123 | 1608 | |
| Trichloroethylene | C2Hydrochloric Acid3 | 20 | 1.477 | 1049 | |
| 1,2,4-Trichlorobenzene | C6H3Chlorine3 | 20 | 1.456 | 1301 | |
| 1-Tridecene | C13H26 | 20 | 0.767 | 1313 | |
| Trimethylene bromide | C3H6Bromine2 | 23.5 | 1.977 | 1144 | |
| Triolein | C3H5(C18H33O2)3 | 20 | 0.92 | 1482 | |
| 1-Undecene | C11H22 | 20 | 0.752 | 1275 | |
| Valeric acid | C4H9Carboxyl | 20 | 0.942 | 1244 | |
| Vinyl acetate, VAc | C4H6O2 | 20 | 0.9317 | 900 | |
| Water | H2O | 25 | 0.997 | 1497 | |
| Xylene (o) | C8H10 | 20 | 0.871 | 1360 | |
| Xylene (m) | C8H10 | 20 | 0.863 | 1340 | |
| Xylene (p) | C8H10 | 20 | 0.86 | 1330 | |
| Citronella oil | 29 | 0.89 | 1076 | ||
| Citric acid 60% | C6H8O7 | 20 | 1686 |
The density measurement of liquids is of great importance in many scientific and industrial applications, as it provides essential information about the composition and properties of liquids. The density of a liquid is a measure of mass per unit volume and can be used to determine a variety of properties.
Accurate knowledge of the density of liquids is crucial for the formulation of chemical recipes, the control of product quality and safety, and the exploration of the physical and chemical properties of liquids. In this context, density determination plays an important role and is a fundamental measurement in this field.
LiquiSonic® is an ultrasonic analyzer for determining the concentration and density of process liquids.