Polymerization
Polymerization is a chemical process in which smaller molecules, known as monomers, join to form larger molecules, called polymers. This process is fundamental to the production of many plastics and other materials. A key aspect of polymerization is the degree of polymerization, which indicates how many monomer units are connected in a polymer molecule. The degree of polymerization significantly affects the physical properties of the resulting polymer, such as strength, flexibility, andtemperature resistance.
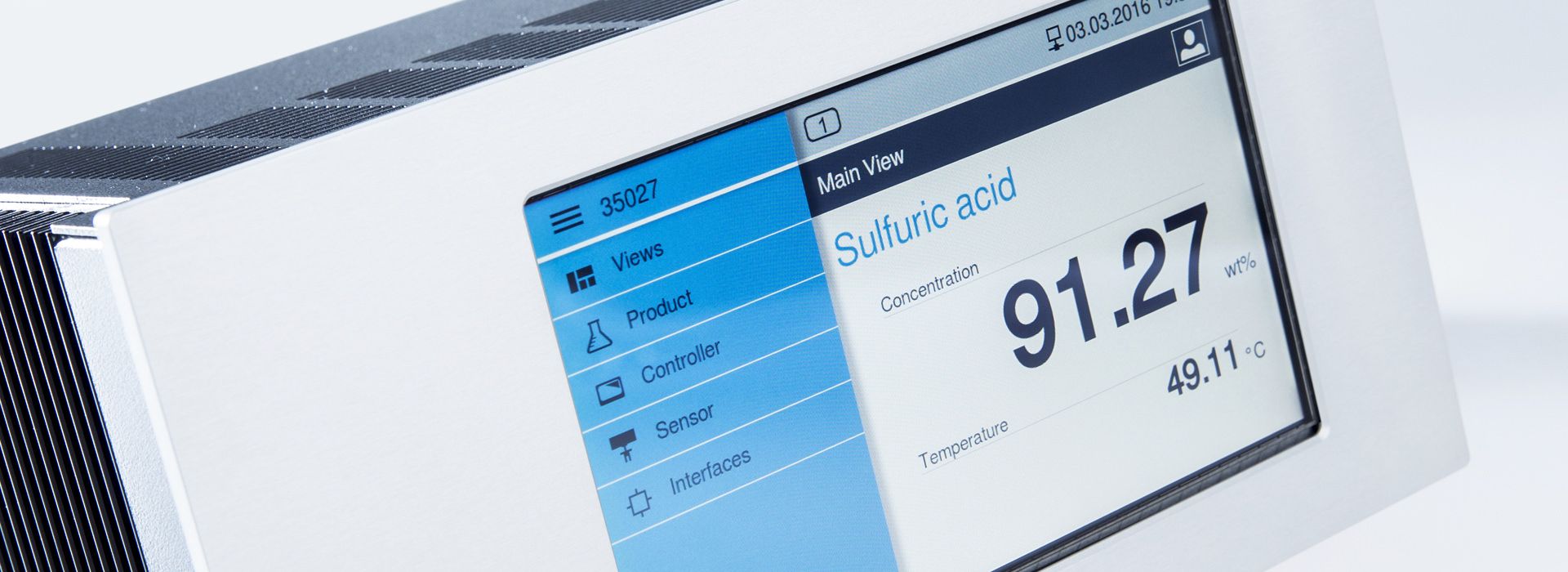
LiquiSonic® Measuring systems in polymerization
LiquiSonic® is an inline analysis system that measures the concentration in polymerization directly in the process without delay. The device is based on the highly precise measurement of the absolute sound velocity and process temperature, allowing the tracking of processes and complex reactions.
The sensor construction of the LiquiSonic® measuring devices enables easy cleaning of the devices, so the process does not have to be interrupted by complex cleaning operations and can run as efficiently as possible.
In the field of polymerization, it offers LiquiSonic® numerous advantages to the user:
- Real-time monitoring: The technology enables continuous monitoring of the polymerization process in real-time. This allows immediate detection of changes and reactions to them, ensuring consistent product quality.
- No sampling required: Since the system measures directly in the process, no manual sampling is required. This minimizes the risk of contamination and process interruptions.
- Robust and low-maintenance technology: LiquiSonic®measuring devices are designed for long-term use in industrial environments. They are resistant to aggressive media and high temperatures, leading to extended service life and reduced maintenance costs.
- Optimization of processes: Through precise monitoring of the polymerization reaction, users can control the process more finely, leading to higher yields and lower production costs.
The LiquiSonic® system can thus be used both in high-precision concentration determination and in phase detection and process monitoring (crystallization). An internal limit value monitoring signals exceedances and shortfalls and sends real-time information to the process control system.
A fast and accurate monitoring of the polymerization, the degree of polymerization, and the concentration of monomers and macromolecules is thus possible. This monitoring ensures that during the entire polymerization of caprolactam to PA6, the optimal product quality is achieved.
Precise knowledge of the course of polymerization and the ratio of monomers to macromolecules is particularly important to minimize product losses and maximize process efficiency. By precisely determining the concentration of monomers and macromolecules throughout the process, the user can ensure that the final product meets the desired specifications.
LiquiSonic® ensures a highly precise analysis of caprolactam concentration with permanent data recording. The measurement system is also successfully used for rapid phase separation between caprolactam and ammonium sulfate.
Sensor construction of LiquiSonic®
The robust sensor construction and the choice of special materials, such as HC2000 or PFA, ensure long process uptime of the system. In addition, SensoTech offers sensors with corresponding ATEX, IECEx, and FM certification.
Through LiquiSonic® the concentration of residual caprolactam (residual monomer) is minimized, thus optimizing plant productivity.
The LiquiSonic® immersion sensors can be easily installed in the supply and transport lines. When installing the LiquiSonic® sensors no bypass is necessary and dead spaces are avoided.
The LiquiSonic® Controller 30 can be connected to up to 4 sensors. This makes it possible to monitor several measuring points simultaneously.
Typical measuring ranges
Concentration range caprolactam: 70 to 100 m%
Temperature range: 80 to 130 °C
Concentration range caprolactam: 0 to 10 m%
Temperature range: 20 to 70 °C
At goods receipt: Concentration range oleum: 0 to 30 m%
Temperature range: 10 to 60 °C
Basics of polymerization
Definition of polymerization
Polymerization is a chemical process in which monomers (individual molecules) are joined to form a macromolecule (polymer).
The determination of conversion in chemical reactions is generally and especially in polymerization reactions of high necessity with regard to process monitoring, process control, and process management.
Just like concentration measurement, the importance of monitoring polymerization in all areas of the economy is currently increasing enormously. High economic effects, such as material and energy savings as well as quality improvements, are possible.
For concentration and conversion measurements, there are a number of measurement methods, such as density measurement, refractive index measurement, conductivity measurement, and the measurement of color, turbidity, and viscosity, all of which have their physical and technological application limits.
The possibility of determining concentrations by measuring the speed of sound has been known for some time and has established itself as a standard measurement method.
Physical principles of polymerization
The propagation speed v of ultrasound in liquids depends on their density and adiabatic compressibility according to the following relationship:
v = speed of sound
ρ = density
βad = adiabatic compressibility
A determining factor for the speed of sound is compressibility. This means that as the speed of sound increases, the density and compressibility can be inversely related. As a result, large differences in the speed of sound can occur even with small density differences. The reverse case is very rare.
The speed of sound is determined by the structure of the substance, i.e., by atomic and molecular groups, isomerism, or chain lengths. This relationship thus provides the opportunity to characterize substances using ultrasound.
The speed of sound v of some selected monomers and polymers at 20 °C is shown in the following table.
The structure of the macromolecule, which is created by the polymerization of monomers, influences the speed of sound, as it is determined by the arrangement of atomic and molecular groups, isomerism, and chain lengths.
For monomer-polymer systems, it is generally true that the differences in the speed of sound between monomer and polymer are primarily determined by the chain length and the degree of branching and cross-linking. The table already clearly shows that the differences between monomer and polymer, and thus between the start and end of the polymerization reaction, can be very large.
Measurement methods in polymerization
To determine the degree of polymerization, various measurement methods are used to monitor the progress and quality of the process. Common methods include viscosity measurements, concentration measurements, gravimetry, and calorimetry.
Problems with viscosity measurement
Viscosity measurements are common, but they can be problematic. In particular, they are influenced by temperature fluctuations, shear rates, and the presence of impurities, which can change the viscosity of the polymer mixture and thus provide inaccurate measurement results. Additionally, viscosity is difficult to measure at very high or very low molecular weights.
The occurrence of impurities can result in unreliable measurement results and then requires an intensive cleaning process, which negatively affects the effectiveness of the process.
Advantages of concentration measurement
Unlike viscosity measurement, concentration measurements are less susceptible to interfering factors. They provide a direct measurement of monomer concentration and are not dependent on the physical properties of the polymers. This leads to more accurate and reliable data on the progress of polymerization.
Processes
Polymerization can occur through various reaction mechanisms, where the monomers react to form longer chains or branched structures, the macromolecules. Polymerizations are classified according to the reaction mechanism into:
- Solution polymerization
- Emulsion polymerization
- Suspension polymerization
- Polycondensation
Depending on the number of copolymers and product-modifying additives, the change in sound velocity shows a characteristic pattern. Typically, the sound velocity of all involved components is determined as a function of temperature to compensate for this later. From the temporal course of the sound velocity, the reaction progress can then be derived and the material conversion calculated.
In the following description, this is explained as an example for the emulsion polymerization of styrene-butadiene latex. The determination of parameters such as concentration, degree of polymerization, etc., is analogous in the other types of polymerization.
Emulsion polymerization of styrene-butadiene latex for the reaction system
In the emulsion polymerization of butadiene-styrene, the individual components and the latexes were examined.
In the following figure, it is shown that the speed of sound of the monomers differs significantly from that of the polymers.
The speed of sound and the concentration are directly related. Furthermore, the degree of polymerization, which represents the proportion of the polymer in the monomer, correlates with the concentration. Therefore, it is possible to determine the concentration and the degree of polymerization using ultrasonic measurement technology. The following figure illustrates this relationship in the polymerization of butadiene-styrene.
In the case of the emulsion polymerization of butadiene and styrene, the degree of polymerization can be determined with an accuracy of 0.1%.
Applications
Based on our experience of over 20 years, a lot of knowledge has accumulated in the field of polymerization, which has been gathered through applications at customer sites and in our company's own technical center. This knowledge flows into new projects, with customer data always being treated confidentially.
During polymerization, not only macromolecules but also monomers come into focus for monitoring, to ensure the exact course of the reaction and the quality of the product.
The following secondary literature is available at SensoTech for different manufacturing processes:
- Optimization of polyamide production
- Optimization of polyurethane production
- Styrene-butadiene latex (SBR) production safe and efficient
The applications investigated so far include:
- Caprolactam polymerization
- Styrene-butadiene latex
- Phenol-formaldehyde resin
- Poly(methyl methacrylate) PMMA
- Polyvinyl acetate PVA
- Polyvinyl chloride PVC
- Polyamide PA
- Polyvinylidene chloride PVdC
- Epoxy resin
- Polystyrene PS
- Polycarbonate PC
- Polyester PE
- Polyethylene
- Formaldehyde-urea resin
- Elastane
- Aldol in acetaldehyde
- Polyurethane PU
- Polysiloxane
- Isoprene rubber IR
- Methyl silicone resin
- Silicone acrylate
- Potassium methylsiliconate
- Silicone resin
- Polysulfide polymer
- Para-phenylene terephthalamide PPTA
- Hindered amine light stabilizers HALS
- Methacrylamide MAA
- Custom compositions
The measuring device LiquiSonic® enables the monitoring and control of different reactions, especially in batch processes. Depending on the method and process fluid, catalytic and enzymatic reactions as well as polymerizations, crystallizations, and mixing processes can be optimized, ensuring the quality of the end product.
For monomer-polymer systems, it is generally true that the differences in sound velocity between monomer and polymer are primarily determined by the chain length and the degree of branching and cross-linking.
The table shows that the differences in sound velocity between monomer and polymer, and thus between the start and end of the polymerization reaction, are very large.
The sound velocity and concentration are directly related. Furthermore, the degree of polymerization, which reflects the polymer content in the monomer, correlates with the concentration. For this reason, the concentration and the degree of polymerization can be determined with the LiquiSonic® measurement technology.
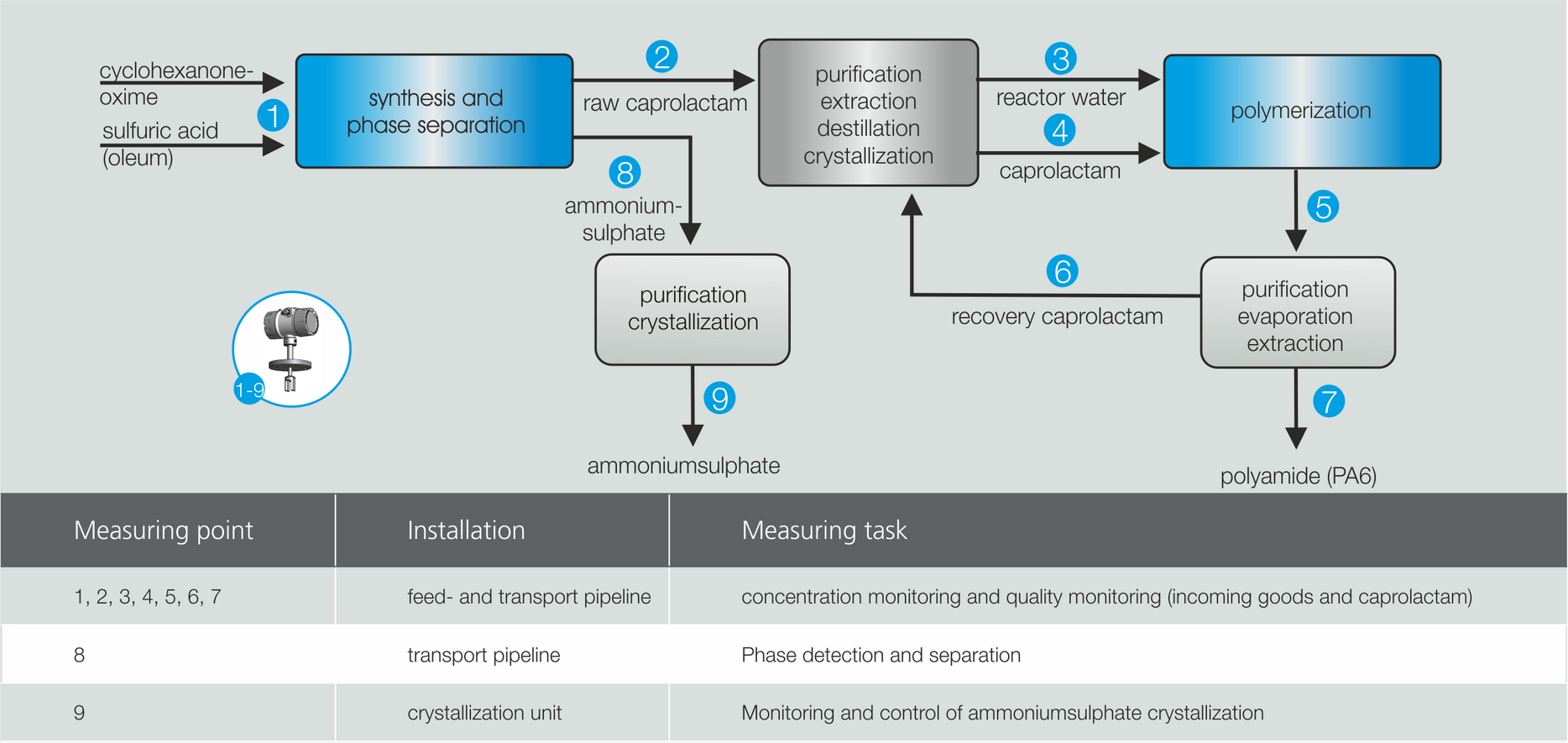
Application example caprolactam production
One of the world's most important polyamides is PA6, known as Perlon, which is produced by the polymerization of the monomer caprolactam (CPL). Due to the complexity of the manufacturing process, it is divided into 4 areas:
- Synthesis of crude caprolactam
- Separation and crystallization of ammonium sulfate
- Purification and processing of crude caprolactam
- Polymerization to PA6
In caprolactam production, cyclohexanone, hydroxylamine, and H are first used2SO4 The base material cyclohexanone oxime is produced. By adding oleum and ammonia, crude caprolactam is generated, which is separated from the ammonium sulfate phase. Subsequently, the monomer caprolactam is purified and concentrated by extraction and crystallization. After polymerization, the polymer is finally separated from the residual monomers and purified.
Macromolecules, polymers, and plastics are ubiquitous products and must meet the highest standards. The processes developed for their manufacture often operate under high process pressures and temperatures. The monitoring and control of these processes must meet the highest safety requirements due to these conditions.